on video What is electricity? - Electricity Explained - (1)
Getting Started
Electricity is all around us--powering technology like our cell phones, computers, lights, soldering irons, and air conditioners. It's tough to escape it in our modern world. Even when you try to escape electricity, it's still at work throughout nature, from the lightning in a thunderstorm to the synapses inside our body. But what exactly is electricity? This is a very complicated question, and as you dig deeper and ask more questions, there really is not a definitive answer, only abstract representations of how electricity interacts with our surroundings.
Electricity is a natural phenomenon that occurs throughout nature and takes many different forms. In this tutorial we'll focus on current electricity: the stuff that powers our electronic gadgets. Our goal is to understand how electricity flows from a power source through wires, lighting up LEDs, spinning motors, and powering our communication devices.
Electricity is briefly defined as the flow of electric charge, but there's so much behind that simple statement. Where do the charges come from? How do we move them? Where do they move to? How does an electric charge cause mechanical motion or make things light up? So many questions! To begin to explain what electricity is we need to zoom way in, beyond the matter and molecules, to the atoms that make up everything we interact with in life.
This tutorial builds on some basic understanding of physics, force, energy, atoms, and [fields](http://en.wikipedia.org/wiki/Field_(physics)) in particular. We'll gloss over the basics of each of those physics concepts, but it may help to consult other sources as well.
Going Atomic
To understand the fundamentals of electricity, we need to begin by focusing in on atoms, one of the basic building blocks of life and matter. Atoms exist in over a hundred different forms as chemical elements like hydrogen, carbon, oxygen, and copper. Atoms of many types can combine to make molecules, which build the matter we can physically see and touch.
Atoms are tiny, stretching at a max to about 300 picometers long (that's 3x10-10 or 0.0000000003 meters). A copper penny (if it actually were made of 100% copper) would have 3.2x1022 atoms (32,000,000,000,000,000,000,000 atoms) of copper inside it.
Even the atom isn't small enough to explain the workings of electricity. We need to dive down one more level and look in on the building blocks of atoms: protons, neutrons, and electrons.
Building Blocks of Atoms
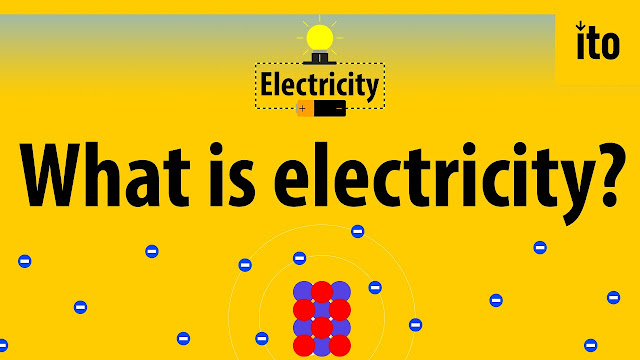
An atom is built with a combination of three distinct particles: electrons, protons, and neutrons. Each atom has a center nucleus, where the protons and neutrons are densely packed together. Surrounding the nucleus are a group of orbiting electrons.
A very simple atom model. It's not to scale but helpful for understanding how an atom is built. A core nucleus of protons and neutrons is surrounded by orbiting electrons.
Every atom must have at least one proton in it. The number of protons in an atom is important, because it defines what chemical element the atom represents. For example, an atom with just one proton is hydrogen, an atom with 29 protons is copper, and an atom with 94 protons is plutonium. This count of protons is called the atom's atomic number.
The proton's nucleus-partner, neutrons, serve an important purpose; they keep the protons in the nucleus and determine the isotope of an atom. They're not critical to our understanding of electricity, so let's not worry about them for this tutorial.
Electrons are critical to the workings of electricity (notice a common theme in their names?) In its most stable, balanced state, an atom will have the same number of electrons as protons. As in the Bohr atom model below, a nucleus with 29 protons (making it a copper atom) is surrounded by an equal number of electrons.
Getting Started
Electricity is all around us--powering technology like our cell phones, computers, lights, soldering irons, and air conditioners. It's tough to escape it in our modern world. Even when you try to escape electricity, it's still at work throughout nature, from the lightning in a thunderstorm to the synapses inside our body. But what exactly is electricity? This is a very complicated question, and as you dig deeper and ask more questions, there really is not a definitive answer, only abstract representations of how electricity interacts with our surroundings.
Electricity is a natural phenomenon that occurs throughout nature and takes many different forms. In this tutorial we'll focus on current electricity: the stuff that powers our electronic gadgets. Our goal is to understand how electricity flows from a power source through wires, lighting up LEDs, spinning motors, and powering our communication devices.
Electricity is briefly defined as the flow of electric charge, but there's so much behind that simple statement. Where do the charges come from? How do we move them? Where do they move to? How does an electric charge cause mechanical motion or make things light up? So many questions! To begin to explain what electricity is we need to zoom way in, beyond the matter and molecules, to the atoms that make up everything we interact with in life.
This tutorial builds on some basic understanding of physics, force, energy, atoms, and [fields](http://en.wikipedia.org/wiki/Field_(physics)) in particular. We'll gloss over the basics of each of those physics concepts, but it may help to consult other sources as well.
Going Atomic
To understand the fundamentals of electricity, we need to begin by focusing in on atoms, one of the basic building blocks of life and matter. Atoms exist in over a hundred different forms as chemical elements like hydrogen, carbon, oxygen, and copper. Atoms of many types can combine to make molecules, which build the matter we can physically see and touch.
Atoms are tiny, stretching at a max to about 300 picometers long (that's 3x10-10 or 0.0000000003 meters). A copper penny (if it actually were made of 100% copper) would have 3.2x1022 atoms (32,000,000,000,000,000,000,000 atoms) of copper inside it.
Even the atom isn't small enough to explain the workings of electricity. We need to dive down one more level and look in on the building blocks of atoms: protons, neutrons, and electrons.
Building Blocks of Atoms
An atom is built with a combination of three distinct particles: electrons, protons, and neutrons. Each atom has a center nucleus, where the protons and neutrons are densely packed together. Surrounding the nucleus are a group of orbiting electrons.
A very simple atom model. It's not to scale but helpful for understanding how an atom is built. A core nucleus of protons and neutrons is surrounded by orbiting electrons.
Every atom must have at least one proton in it. The number of protons in an atom is important, because it defines what chemical element the atom represents. For example, an atom with just one proton is hydrogen, an atom with 29 protons is copper, and an atom with 94 protons is plutonium. This count of protons is called the atom's atomic number.
The proton's nucleus-partner, neutrons, serve an important purpose; they keep the protons in the nucleus and determine the isotope of an atom. They're not critical to our understanding of electricity, so let's not worry about them for this tutorial.
Electrons are critical to the workings of electricity (notice a common theme in their names?) In its most stable, balanced state, an atom will have the same number of electrons as protons. As in the Bohr atom model below, a nucleus with 29 protons (making it a copper atom) is surrounded by an equal number of electrons.
Getting Started
Electricity is all around us--powering technology like our cell phones, computers, lights, soldering irons, and air conditioners. It's tough to escape it in our modern world. Even when you try to escape electricity, it's still at work throughout nature, from the lightning in a thunderstorm to the synapses inside our body. But what exactly is electricity? This is a very complicated question, and as you dig deeper and ask more questions, there really is not a definitive answer, only abstract representations of how electricity interacts with our surroundings.
Electricity is a natural phenomenon that occurs throughout nature and takes many different forms. In this tutorial we'll focus on current electricity: the stuff that powers our electronic gadgets. Our goal is to understand how electricity flows from a power source through wires, lighting up LEDs, spinning motors, and powering our communication devices.
Electricity is briefly defined as the flow of electric charge, but there's so much behind that simple statement. Where do the charges come from? How do we move them? Where do they move to? How does an electric charge cause mechanical motion or make things light up? So many questions! To begin to explain what electricity is we need to zoom way in, beyond the matter and molecules, to the atoms that make up everything we interact with in life.
This tutorial builds on some basic understanding of physics, force, energy, atoms, and [fields](http://en.wikipedia.org/wiki/Field_(physics)) in particular. We'll gloss over the basics of each of those physics concepts, but it may help to consult other sources as well.
Going Atomic
To understand the fundamentals of electricity, we need to begin by focusing in on atoms, one of the basic building blocks of life and matter. Atoms exist in over a hundred different forms as chemical elements like hydrogen, carbon, oxygen, and copper. Atoms of many types can combine to make molecules, which build the matter we can physically see and touch.
Atoms are tiny, stretching at a max to about 300 picometers long (that's 3x10-10 or 0.0000000003 meters). A copper penny (if it actually were made of 100% copper) would have 3.2x1022 atoms (32,000,000,000,000,000,000,000 atoms) of copper inside it.
Even the atom isn't small enough to explain the workings of electricity. We need to dive down one more level and look in on the building blocks of atoms: protons, neutrons, and electrons.
Building Blocks of Atoms
An atom is built with a combination of three distinct particles: electrons, protons, and neutrons. Each atom has a center nucleus, where the protons and neutrons are densely packed together. Surrounding the nucleus are a group of orbiting electrons.
A very simple atom model. It's not to scale but helpful for understanding how an atom is built. A core nucleus of protons and neutrons is surrounded by orbiting electrons.
Every atom must have at least one proton in it. The number of protons in an atom is important, because it defines what chemical element the atom represents. For example, an atom with just one proton is hydrogen, an atom with 29 protons is copper, and an atom with 94 protons is plutonium. This count of protons is called the atom's atomic number.
The proton's nucleus-partner, neutrons, serve an important purpose; they keep the protons in the nucleus and determine the isotope of an atom. They're not critical to our understanding of electricity, so let's not worry about them for this tutorial.
Electrons are critical to the workings of electricity (notice a common theme in their names?) In its most stable, balanced state, an atom will have the same number of electrons as protons. As in the Bohr atom model below, a nucleus with 29 protons (making it a copper atom) is surrounded by an equal number of electrons.
Getting Started
Electricity is all around us--powering technology like our cell phones, computers, lights, soldering irons, and air conditioners. It's tough to escape it in our modern world. Even when you try to escape electricity, it's still at work throughout nature, from the lightning in a thunderstorm to the synapses inside our body. But what exactly is electricity? This is a very complicated question, and as you dig deeper and ask more questions, there really is not a definitive answer, only abstract representations of how electricity interacts with our surroundings.
Electricity is a natural phenomenon that occurs throughout nature and takes many different forms. In this tutorial we'll focus on current electricity: the stuff that powers our electronic gadgets. Our goal is to understand how electricity flows from a power source through wires, lighting up LEDs, spinning motors, and powering our communication devices.
Electricity is briefly defined as the flow of electric charge, but there's so much behind that simple statement. Where do the charges come from? How do we move them? Where do they move to? How does an electric charge cause mechanical motion or make things light up? So many questions! To begin to explain what electricity is we need to zoom way in, beyond the matter and molecules, to the atoms that make up everything we interact with in life.
This tutorial builds on some basic understanding of physics, force, energy, atoms, and [fields](http://en.wikipedia.org/wiki/Field_(physics)) in particular. We'll gloss over the basics of each of those physics concepts, but it may help to consult other sources as well.
Going Atomic
To understand the fundamentals of electricity, we need to begin by focusing in on atoms, one of the basic building blocks of life and matter. Atoms exist in over a hundred different forms as chemical elements like hydrogen, carbon, oxygen, and copper. Atoms of many types can combine to make molecules, which build the matter we can physically see and touch.
Atoms are tiny, stretching at a max to about 300 picometers long (that's 3x10-10 or 0.0000000003 meters). A copper penny (if it actually were made of 100% copper) would have 3.2x1022 atoms (32,000,000,000,000,000,000,000 atoms) of copper inside it.
Even the atom isn't small enough to explain the workings of electricity. We need to dive down one more level and look in on the building blocks of atoms: protons, neutrons, and electrons.
Building Blocks of Atoms
An atom is built with a combination of three distinct particles: electrons, protons, and neutrons. Each atom has a center nucleus, where the protons and neutrons are densely packed together. Surrounding the nucleus are a group of orbiting electrons.
A very simple atom model. It's not to scale but helpful for understanding how an atom is built. A core nucleus of protons and neutrons is surrounded by orbiting electrons.
Every atom must have at least one proton in it. The number of protons in an atom is important, because it defines what chemical element the atom represents. For example, an atom with just one proton is hydrogen, an atom with 29 protons is copper, and an atom with 94 protons is plutonium. This count of protons is called the atom's atomic number.
The proton's nucleus-partner, neutrons, serve an important purpose; they keep the protons in the nucleus and determine the isotope of an atom. They're not critical to our understanding of electricity, so let's not worry about them for this tutorial.
Electrons are critical to the workings of electricity (notice a common theme in their names?) In its most stable, balanced state, an atom will have the same number of electrons as protons. As in the Bohr atom model below, a nucleus with 29 protons (making it a copper atom) is surrounded by an equal number of electrons.







No comments