on video How A Car Battery Works - basic working principle
Share
Share
Print
How does a car battery work and how is it constructed?
Battery Basics
June 27, 2018
The traditional function of the battery in the engine compartment is well known: Without the battery the vehicle cannot be started. In addition to the starter motor, the spark plugs, glow plugs, lights and electronic applications all require electrical energy. But how is a battery constructed and how does it work?
Lead-acid batteries: Components and structure
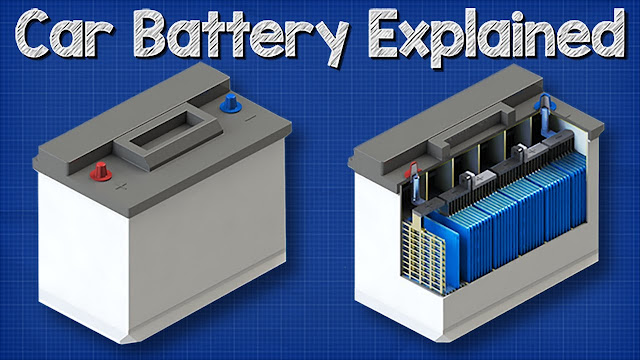
Many drivers become aware of the heavy weight of car batteries when they buy a new one. Weights from about 10.5 kg, up to 30 kg are possible. The reason for this is the lead plates in the battery cells.
Components and structure of a battery cell
Positive electrode:
Positive plate: In a lead-acid battery, the positively charged plate (active material) consists of lead oxide (PbO2) which is immersed in an electrolyte.
Positive grid: The positive grid consists of a lead alloy and is used to hold the active material and as a current collector.
Negative electrode:
Negative plate: The negatively charged plate (active material) consists of pure lead (Pb), which is also immersed in an electrolyte.
Negative plate: Like the positive plate, this also consists of a lead alloy and serves the same purpose.
The electrodes with different charges are separated by a separator bag.
The electrolyte is a mixture of sulfuric acid (H2SO4) and distilled water. This electrolyte can be in liquid form (as in conventional wet batteries or in the enhanced EFB technology), in gel form, or bound in a glass mat (as in AGM technology for newer start-stop applications).
Several positive electrodes form a positive plate set and several negative electrodes form a negative plate set. Together, a negative and a positive plate set form a plate block. A plate block is a battery cell.
Share
Share
Print
How does a car battery work and how is it constructed?
Battery Basics
June 27, 2018
The traditional function of the battery in the engine compartment is well known: Without the battery the vehicle cannot be started. In addition to the starter motor, the spark plugs, glow plugs, lights and electronic applications all require electrical energy. But how is a battery constructed and how does it work?
Lead-acid batteries: Components and structure
Many drivers become aware of the heavy weight of car batteries when they buy a new one. Weights from about 10.5 kg, up to 30 kg are possible. The reason for this is the lead plates in the battery cells.
Components and structure of a battery cell
Positive electrode:
Positive plate: In a lead-acid battery, the positively charged plate (active material) consists of lead oxide (PbO2) which is immersed in an electrolyte.
Positive grid: The positive grid consists of a lead alloy and is used to hold the active material and as a current collector.
Negative electrode:
Negative plate: The negatively charged plate (active material) consists of pure lead (Pb), which is also immersed in an electrolyte.
Negative plate: Like the positive plate, this also consists of a lead alloy and serves the same purpose.
The electrodes with different charges are separated by a separator bag.
The electrolyte is a mixture of sulfuric acid (H2SO4) and distilled water. This electrolyte can be in liquid form (as in conventional wet batteries or in the enhanced EFB technology), in gel form, or bound in a glass mat (as in AGM technology for newer start-stop applications).
Several positive electrodes form a positive plate set and several negative electrodes form a negative plate set. Together, a negative and a positive plate set form a plate block. A plate block is a battery cell.







No comments